Portal Signup
Advanta's QR portal signup is simple to use with our easy to follow video guide.
* Turnaround time starts when sample is received at the laboratory.
Advanta Genetics offers
Furthermore, because viruses like SARS-CoV-2 (COVID-19) continuously evolve and change their genetic code, Advanta scientists are now performing
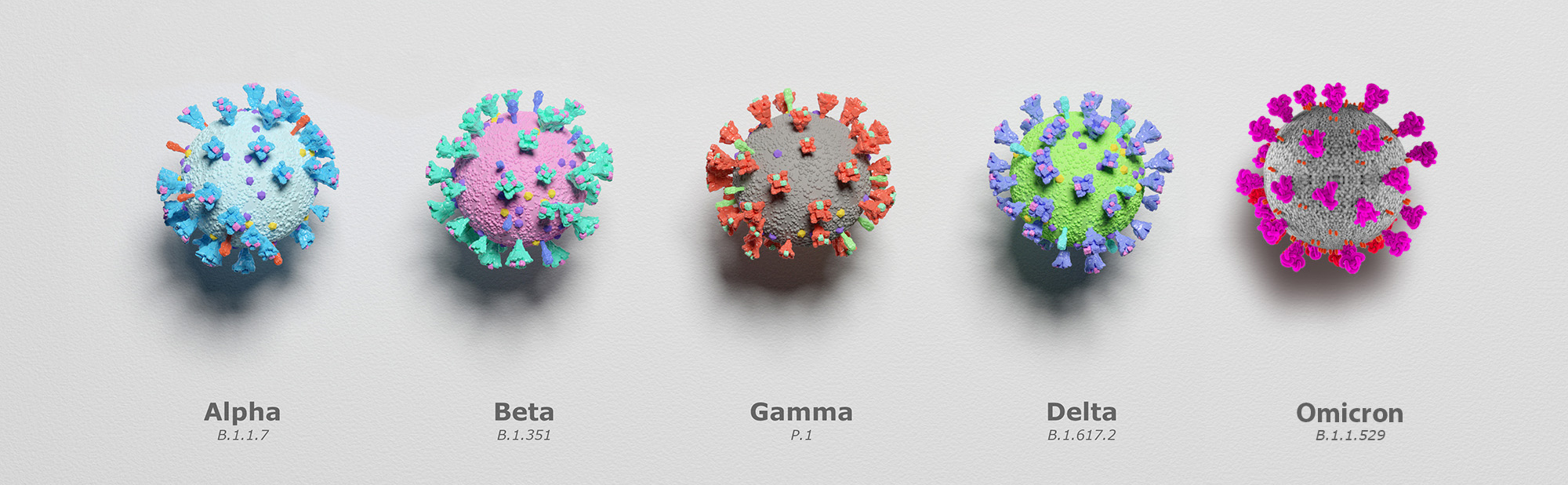
To date Advanta has performed close to a million COVID-19 PCR test. For more information on COVID-19 testing or to speak with one of our scientists, reach us at 903-805-8855 or email us at info@aalabs.com.
Need Covid-19 results fast? Not only is AdvantaBridge simple to use, it's easy to access from anywhere. Contact us to sign up today.
Advanta's QR portal signup is simple to use with our easy to follow video guide.
Advanta Genetics offers same day COVID-19 results if the sample is received by 2:00 pm.
Our portal, AdvantaBridge, is a secure, user-friendly tool for downloading results on the go.
Flying somewhere? Our reports feature a QR code for fast, mobile-friendly check-in.
This nucleic acid amplification test was developed, and its performance characteristics determined with polymerase chain reaction (PCR) by Advanta Genetics. This is a laboratory developed test and has not been cleared by the FDA. Advanta Genetics is regulated by CLIA as qualified to perform high complexity testing and is accredited by the College of America Pathologist (CAP). This test is for clinical purpose and should not be regarded as investigational or for research purposes.